Abstract
Introduction:
The role of prophylactic treatment for central nervous system (CNS) relapse in DLBCL is not clearly established. The selection of patients (pts) and the optimal regimens, including choice of agents, timing of administration, dosing, route, etc., remain unclear. In an effort to develop a uniform approach to this strategy, we have reviewed the practice of CNS ppx in DLBCL at Memorial Sloan Kettering during 2004-2014.
Methods:
We retrospectively reviewed 1) all newly diagnosed pts with DLBCL from 2004-2014 who received CNS ppx, and 2) all pts with DLBCL who developed CNS relapse/progression during the same period. All pts were treated with rituximab-based chemotherapy. Prophylactic agents included methotrexate (MTX) and/or cytarabine (ara-C). Pts with CNS or ocular involvement upon presentation were excluded. All data was compiled and analyzed using SPSS (IBM).
Results:
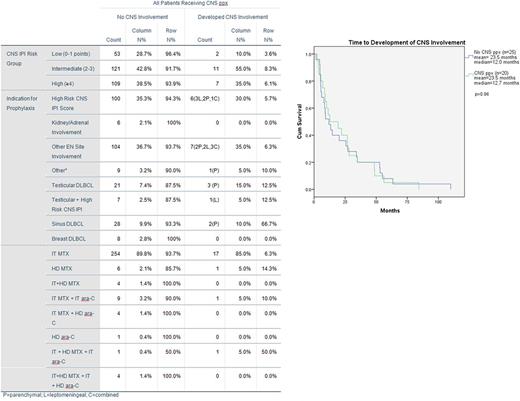
Three hundred and three pts received CNS ppx. CNS-IPI (Schmitz et al. J Clin Oncol, 2016) classified pts as low (18.2%), intermediate (43.6%), or high risk (38.2%) for CNS relapse. Seventy-six percent of pts presented with stage IV disease and a mean of 2.1 EN sites, frequently involving bone (53.8%) (vertebrae specifically, 33.7%), lung/pleura (20.1%), GI tract (18.2%), sinuses (17.5%), testes (10.5%) and others.
Thirty-five percent of pts had high risk CNS IPI scores and received ppx. Most frequently, pts received ppx due to the presence of EN disease (36.6%), and of these pts 66.4% had multiple EN sites involved. CNS ppx was also administered to pts with primary testicular (24), sinus (30), and breast (8) DLBCL in addition to pts with isolated bone (21) vertebrae (14), and GI tract (5) lesions. The remaining 3.6% of pts received ppx for other reasons including high risk molecular profiles or bulky disease.
Intrathecal (IT) MTX alone was administered to 271 of 303 prophylaxed pts, and 17 (5.9%) developed CNS involvement. High dose intravenous (HD) MTX alone was given to 7 pts; 1 developed CNS involvement. IT MTX and IT ara-C were given to 10 pts; 1 developed CNS involvement. IT and HD MTX plus IT ara-C were given to 2 pts; 1 developed CNS involvement. Additional combinations can been reviewed in Table 1.
Twenty pts (6.6%) failed CNS ppx, with a median (mdn) time from diagnosis to detection of CNS involvement of 12.7 months. No pts developed CNS disease while on first-line therapy. Of 20 pts, 11 had isolated CNS relapses (mdn= 8.9 months). 7 relapsed systemically with CNS involvement (mdn =26.8 months). Two had primary refractory disease with CNS involvement detected at 10.2 and 12.7 months. The difference in time to CNS relapse based on involvement pattern (leptomeningeal, parenchymal or both) or the context of CNS involvement (isolated CNS relapse vs systemic relapse with CNS involvement) was not statistically significant. The pt treated with HD and IT MTX and IT ara-C relapsed in the CNS at 3.5 months, significantly earlier than pts treated with IT MTX (mdn 12.7 mo, p=0.02), but notably received only 3 cycles of R-CHOP due to wound dehiscence.
There were 776 DLBCL pts from the same time period who were not treated with ppx, and 25 (3.2%) developed CNS involvement. One pt relapsed after 48d during R-CHOP. Three additional pts had primary refractory disease with CNS involvement at a median of 5.2 months. Fifteen had isolated CNS relapses (mdn 8.5 months), and 6 had systemic relapses with CNS involvement (mdn 12.0 months). There was no significant difference in the time to CNS relapse comparing pts who received CNS ppx to pts who did not.
Conclusions:
In our study, pts with DLBCL at high risk for CNS relapse treated with rituximab-based chemotherapy developed CNS involvement after systemic therapy was complete. Administering CNS ppx concurrently with R-CHOP poses many challenges, often resulting in dose adjustments or delays in treatment. Our results suggest that CNS ppx can be delayed until the completion of R-CHOP without compromising the effects of ppx.
Moskowitz: Seattle Genetics: Consultancy, Other: Ad Board, Research Funding; Genentech BioOncology: Consultancy; Pharmacyclics: Research Funding; Celgene: Consultancy; Merck: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal